Beyond vaccines, beyond checkpoint inhibitors, chimeric antigen receptor T-cells (CAR T-cells) are the latest form of cancer therapies aimed at reestablishing the body’s immune response to tumors. Like the Chimera of Greek mythology, a hybrid creature composed of more than one animal, CAR T-cells are molecules engineered in the laboratory using a hybrid of proteins grafted onto a patient’s T-cells. The hybrid assembly allows the CAR T-cells to carry out multiple specific functions. This engineering allows CAR T-cells to recognize specific proteins, or antigens, present on the surface of targeted cancer cells, allowing the CAR T-cells to become activated and destroy the tumor.
“CAR T-cells are among the most promising approaches to fighting cancer, especially blood cancers, through the development of adoptive cell transfer therapies,” said Yair Levy, MD, medical director of hematologic malignancy clinical research at Baylor University Medical Center, part of Baylor Scott & White Health. “They represent a dynamic new line of therapies for blood cancers that have repeatedly relapsed after intensive chemotherapy or have simply failed to respond to standard therapies,” Dr. Levy said.
Other cancer immune therapies, such as vaccines derived from a patient’s dendritic cells or checkpoint inhibitors that bypass the immune system’s natural throttles, rely on reawakening the body’s own immune system to seek out and destroy cancer. In contrast, CAR T-cells involve adoptive transfer of effector cells made outside the body, which identify and are targeted specifically against the cancer cells. They may propagate, expand and persist once they are injected into the patient. “With the tools of molecular biology, we can create lymphocytes with properties that never existed before in the course of evolution,” Dr. Levy said. “Some CAR T-cells have shown results that are nothing short of spectacular.”
Beginning of New Chimeric Antigen Receptor T-cell (CAR T-cell) Clinical Trials at Baylor University Medical Center
On Sept. 1, 2016, Baylor University Medical Center began clinical trials involving CAR T-cells targeted at acute lymphoblastic leukemia (ALL) and mantle cell lymphoma (MCL). Houston Holmes, MD, the hematologist on the medical staff at Baylor University Medical Center, serves as principal investigator for these studies. ALL is a cancer that starts from immature forms of white blood cells called lymphocytes in the bone marrow, where new blood cells are made. Acute leukemia invades the blood quickly and can involve the lymph nodes, liver, spleen, brain and spinal cord. MCL is another lymphoid cancer considered treatable, but incurable, with standard therapies. It initially responds to most treatments, but when it recurs, it is quite difficult to treat. Both ALL and MCL are B-cell malignancies that express an antigen called CD19. CAR T-cells in these clinical trials are modified lymphocytes with artificial T-cell receptors specifically engineered to target cancer cells that produce CD19.
How Chimeric Antigen Receptor T-cells (CAR T-cells) Work Against the Cancer
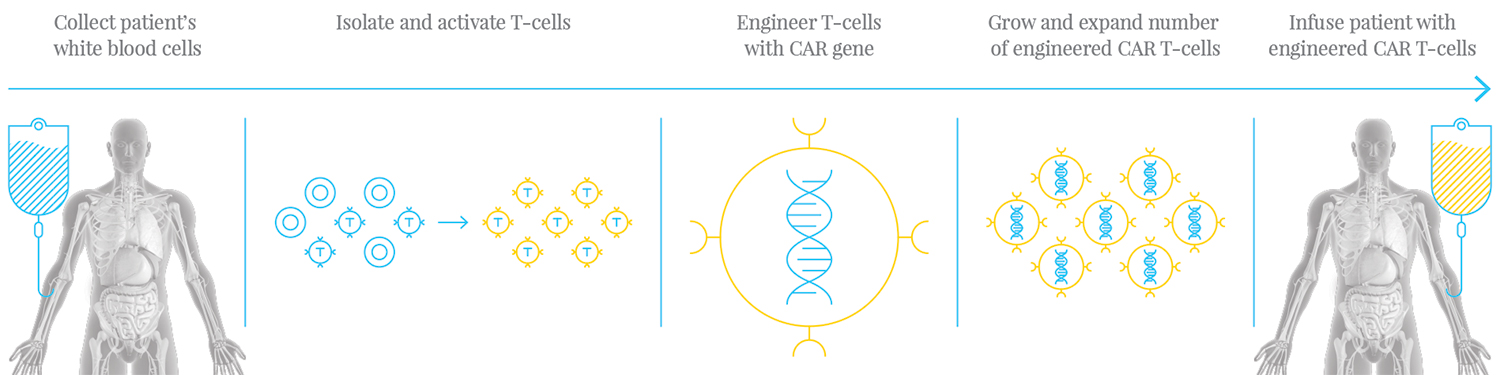
Cancer cells produce antigens that can mediate the normal response of a patient’s immune system, allowing the tumor to progressively grow. Unlike acute infections, cancer growth is initially hidden to the immune system, as T-cells are restrained by mechanisms that limit the immune response to the tumors. “Although the body’s own T-cells are aware of the cancer, they do not activate an antitumor response; they are tolerant,” explained Dr. Levy. T-cells need a secondary stimulation to be activated against the cancer. In the engineering of CAR T-cells, the T-cells are modified with artificial T-cell receptors, producing a second costimulatory signal that activates them against the cancer. “We can take antibodies, manipulate them molecularly, insert them into T-cell lymphocytes and give them a new recognition that they never had before, a recognition based on an antibody,” Dr. Levy said. The CAR T-cells pursue cancer cells that contain specificity to the receptor that was grafted, such as CD19. “CAR T-cells offer a targeted approach to cancer treatment, and especially in blood cancers,” Dr. Levy said. “These are antibodies that can recognize molecules on blood cancers and therefore specifically target them.”