It is easy to envision the immune system as a “police force” that is separate from the organ systems and designed to only respond to threats. However, it is becoming increasingly clear that tissue-resident immune cells are more involved in organ homeostasis than previously thought. Recent work from researchers at Baylor Scott & White Research Institute (BSWRI) has shed light on the alternative functions of immune cells in the skin.
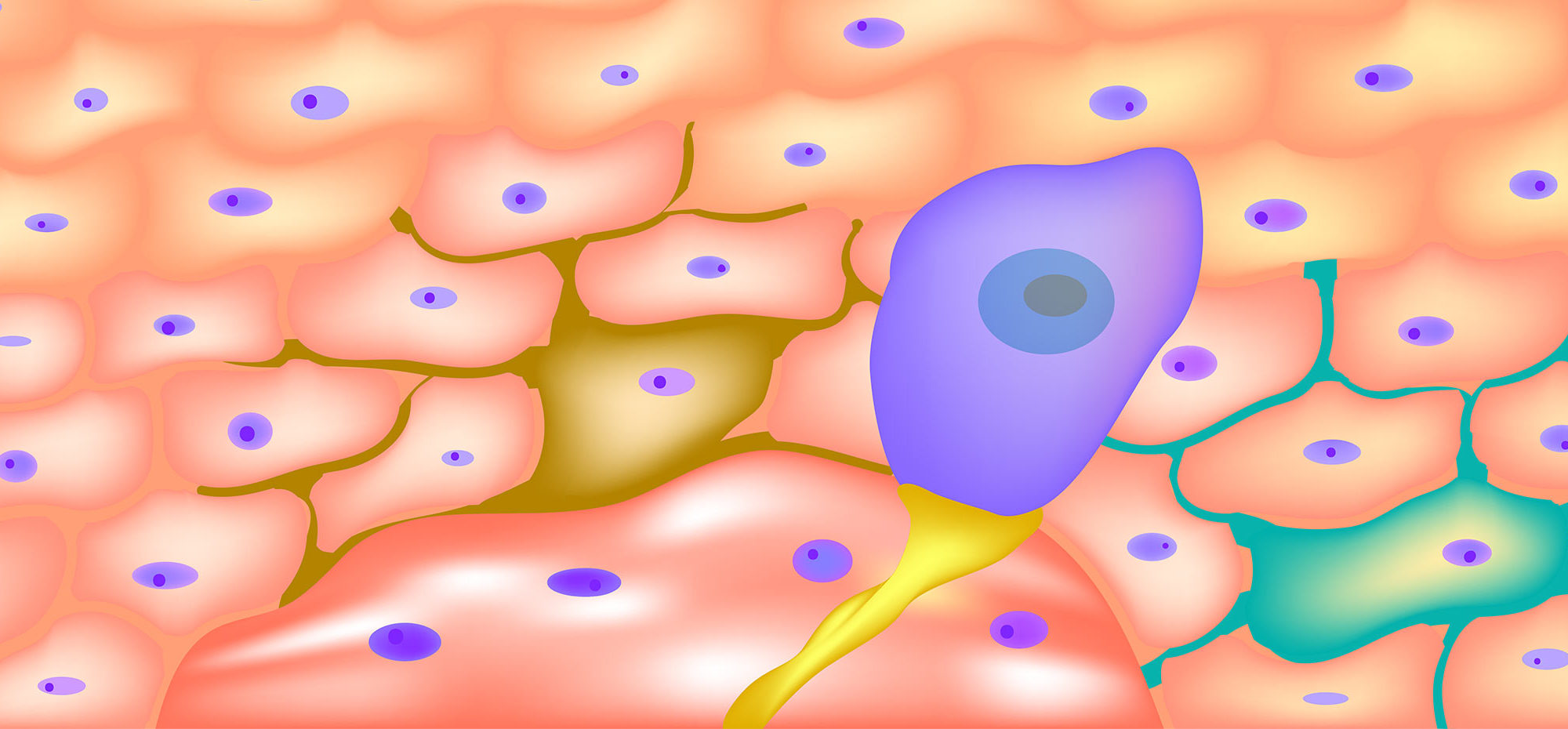
Langerhans cells (LCs), which are specialized antigen-presenting cells, reside in the epidermis and participate in the skin’s immune response to external antigens. Upon antigen stimulation, LCs migrate to lymph nodes to activate T cells and initiate immune responses. However, LCs may also play a deeper role in skin homeostasis by interacting with keratinocytes (KCs) and dendritic epidermal T cells (DETCs), the other main epidermal cell types. Prior work has shown that KCs, which constitute 95% of the epidermis, can use cytokines and other signals to regulate LC function. However, it was not known whether LCs are active participants in the epidermal cell milieu or are just passive recipients of signals from the environment.
BSWRI addressed this question in a study published in the January 20, 2020 edition of PLoS ONE. They research team used transcriptomic profiling to test whether removing LCs from the epidermis could impact the functional status of KCs and DETCs. To eliminate the LCs, they used a mouse strain that couples the gene for Langerin to the expression of diphtheria toxin, which specifically kills all epidermal LCs. Using flow cytometry and RNA sequencing (RNASeq) for cell-type specific transcriptomic profiling, they showed that the absence of LCs changes gene expression in KCs and DETCs. Pathway analysis indicated that loss of LCs increased the expression of genes associated with cell adhesion, ribosome and RNA biogenesis, autophagy, response to infection, and signal transduction in both the KCs and DETCs. Therefore, the LCs may exert a common regulatory influence over both the KCs and DETCs.
This news indicates that LCs are not merely passive receivers of information from the cellular environment, but also act to shape the cellular landscape of the skin. It also adds support to the theory that cells of the immune system can be more integrated into the tissue microenvironment than was previously appreciated.
The idea that LCs regulate tissue homeostasis is supported by results from other organs. For instance, the microglia, which are macrophage-like cells in the brain, are also involved in synapse pruning. Notably, in the current study, nerve growth factor (Ngf) was the mRNA most upregulated by loss of LCs. Whether LCs also participate in neuronal homeostasis in the epidermis remains unknown. They also note that it remains unknown whether the observed changes in mRNA profiles are due exclusively to transcription or whether horizontal transfer of RNA among cells also plays a role.
Important next steps to this work include identifying the factors involved in alerting the epidermal cells to the presence of LCs. Additionally, there is opportunity to explore if short-term loss of LCs is sufficient to alert the tissue to their absence. By understanding the full scope of LC function, researchers may be able to predict outcomes in immune-related diseases of the skin.