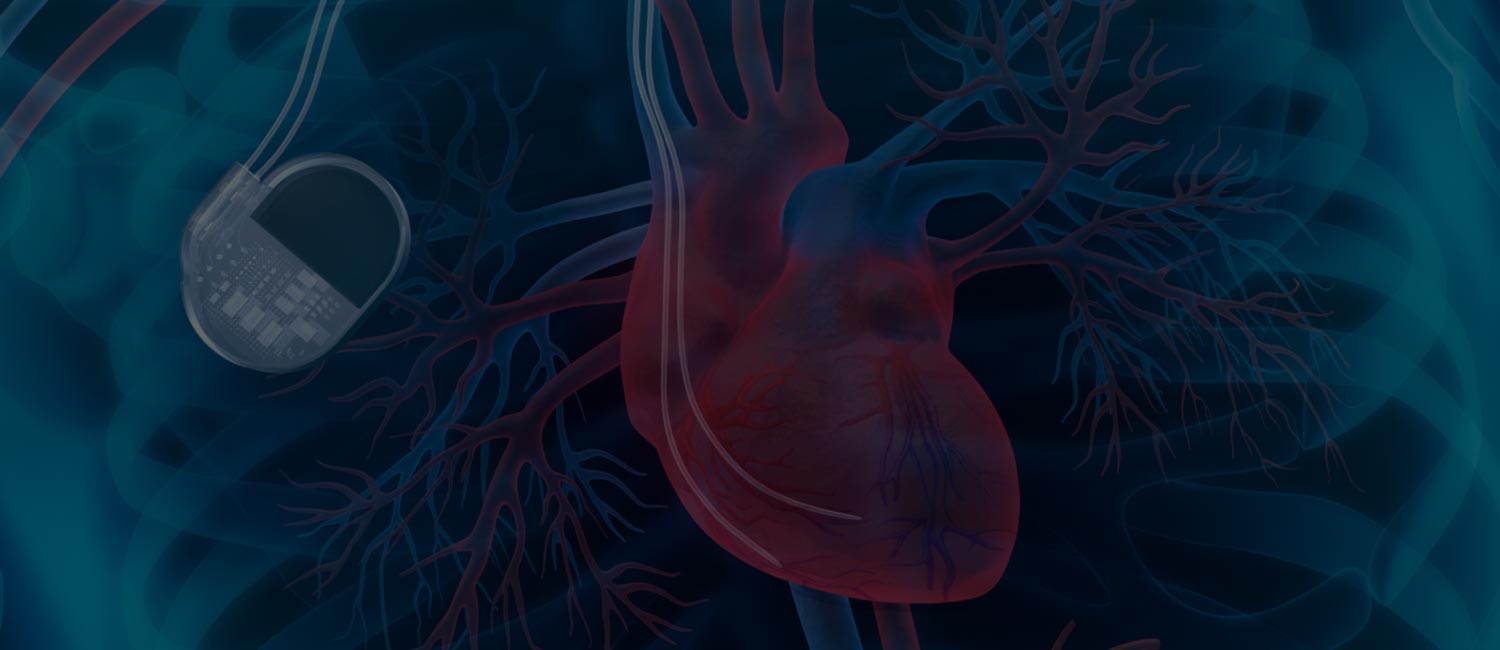
Baylor Scott & White The Heart Hospital – Plano is the only site in Dallas-Fort Worth currently enrolling participants in the SOLVE CRT trial. This innovative trial is evaluating a new cardiac resynchronization therapy (CRT) system that delivers pacing stimulation from inside the left ventricle – possibly eliminating the need for traditional left ventricular lead placement.
In patients with heart failure where the left ventricle is failing, CRT – also known as a biventricular defibrillator – can have a significant benefit. However, in approximately 30 percent of potential candidates, the lead placed on the left ventricle fails to work, usually due to unfavorable vein anatomy, unacceptable phrenic nerve stimulation when activated, or pacing thresholds too high to stimulate the heart effectively.
“For those people who meet the criteria, a biventricular defibrillator has a profound effect on outcome, but not everybody can get the most important piece, which is the left ventricular lead,” said Steven Kindsvater, MD, Vice Chairman, Cardiovascular Medicine at Baylor Scott & White The Heart Hospital – Plano. “That means one-third of people can’t get a device for heart failure who would otherwise benefit.”

Through the SOLVE CRT trial, investigators will evaluate a new implantable cardiac system that provides left ventricular endocardial pacing stimulation in conjunction with a previously placed traditional pacemaker, defibrillator, or CRT device with a nonfunctional left ventricular lead.
The system uses a tiny electrode placed inside the left ventricle chamber that wirelessly connects to an external transmitter placed under the skin. The transmitter works by sensing when the right ventricular lead paces, then sends an ultrasound pulse in 1-3 msec to the left ventricular electrode, enabling true biventricular endocardial pacing to occur.
“This new device takes those individuals who can’t get a biventricular defibrillator and potentially allows them to receive appropriate heart failure therapy because the left ventricular lead placement is no longer dependent on patient anatomy,” Dr. Kindsvater said. “We’re able to replace the left ventricular lead with an electrode that’s about the size of a BB and is put in from the inside of the chamber, instead of from the veins on the outside.”
Potential candidates for the trial include anyone who has regular indications for CRT but either received a device that did not work or did not receive a device at all because of their anatomy.
“For patients with heart failure who get biventricular defibrillators that are either nonfunctional or dysfunctional, there may be another option for the first time,” Dr. Kindsvater said. “If a patient isn’t currently benefiting from a biventricular defibrillator, now there is the potential to provide a significant benefit to these individuals without the need for open heart surgery.”
If the new system is shown to be safe and effective, the trial provides the potential for more patients to have the opportunity to receive CRT and its benefits, including feeling better, living longer and making the heart function better. As part of the trial, Baylor Scott & White Heart – Plano has the unique opportunity to be involved in advancing care for these patients.
“We’re one of only a few sites in the United States to evaluate this technology,” Dr. Kindsvater said. “There is a lot of excitement among the electrophysiology community about this study, and it’s exciting for Baylor Scott & White Heart – Plano to be a part of it.”