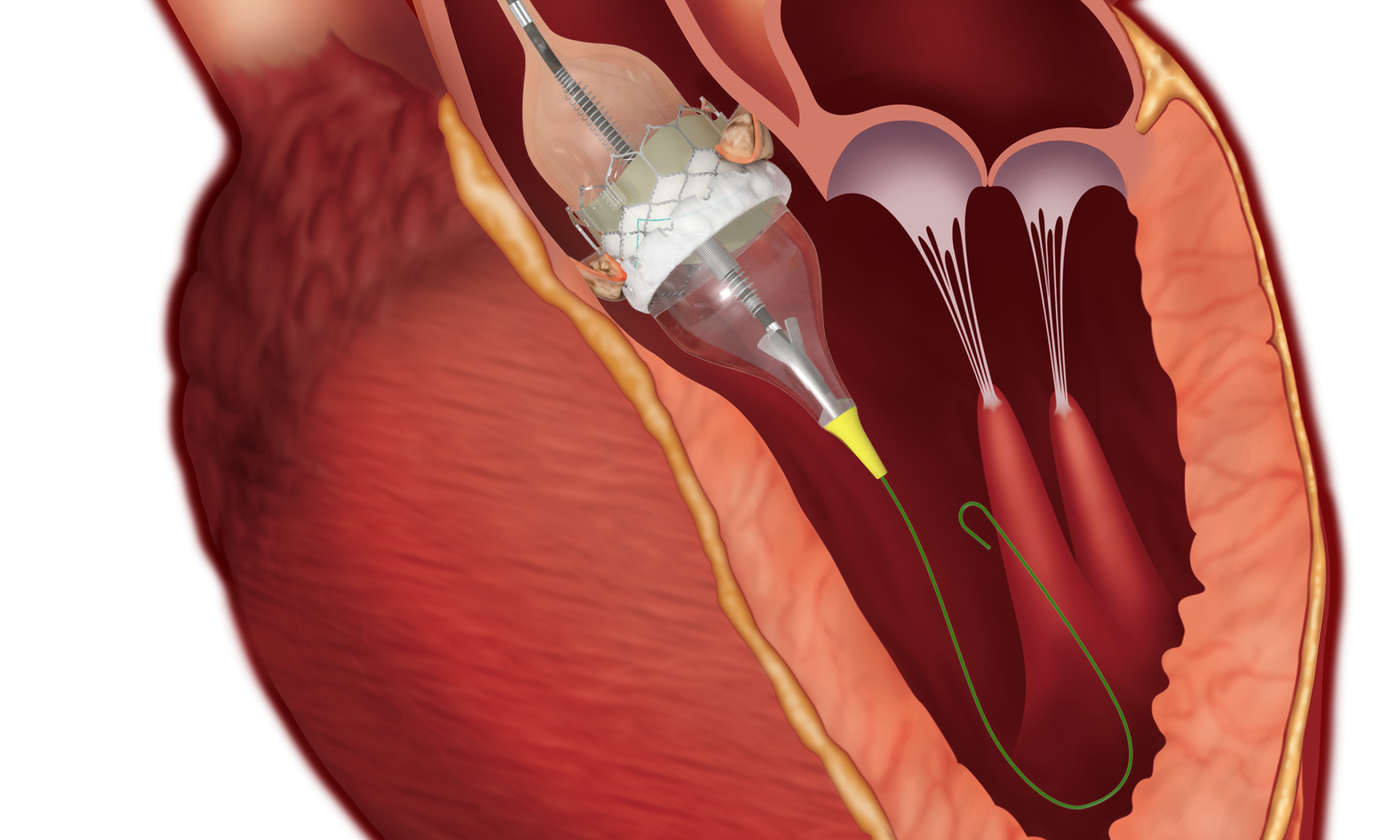
Outcomes from Partner 3, a clinical trial evaluating transcatheter aortic valve replacement, or TAVR, compared to traditional surgical repair for low risk patients with aortic stenosis were recently published in The New England Journal of Medicine.
Baylor Scott & White was the top US enroller in the trial. The results from Partner 3 strongly favored TAVR with a 46% decrease in the rate of the primary endpoint at one year, which met the trial criteria of both non-inferiority and superiority. All components of the primary endpoint – death, stroke and re-hospitalization – favored TAVR, with a number of other outcomes also favoring the procedure, including a shorter hospital stay, less irregular heart rhythm, and less bleeding. As with previous evaluation of TAVR, in this procedure, the placement of a new heart valve was completed by threading it through a blood vessel in the leg up to the heart.
“Based on these results, TAVR should also be considered as a viable option in patients at low surgical risk,” said the national co-principal investigator, Michael Mack, MD, Medical Director of Cardiovascular Surgery, BSWH and Chairman of Baylor Scott & White The Heart Hospital Plano Research Center.
Dr. Mack led the team at Baylor Scott & White The Heart Hospital – Plano, which was a primary site for Partner 3. Together with researchers Baylor Scott & White Medical Center – Temple, the two sites successfully collaborated to identify eligible participants and earn the distinction as the highest enrolling site in the country.
Aortic stenosis, a condition where calcium buildup causes a blockage on the main heart valve, remains one of the most common and most serious valve disease problems, particularly in the elderly. Prior to TAVR, open heart surgery was considered the “gold standard” method of treatment to repair the blocked valve. These findings, together with the full body of research behind TAVR, present a promising new alternative for patients regardless of their level of surgical risk.
Baylor Scott & White’s history with TAVR goes back nearly 20 years. In 2001, research began in Leipzig, Germany and investigators at The Heart Hospital Plano worked with German colleagues to test the approach. TAVR was performed in humans as part of early clinical trials less than five years later. In the US to date, about 60,000 patients receive TAVR – generally high or moderate risk patients – and 25,000 patients undergo traditional open-heart surgery annually. Baylor Scott & White is the largest provider of this innovative therapy in the state of Texas and treats about 800 TAVR patients on average per year across its system.
Partner 3 is the fifth study in a series of trials evaluating TAVR compared with open heart surgery in patients with various degrees of surgical risk. Preceding studies led to FDA approval of TAVR use in patients deemed high or moderate risk for complications or adverse outcomes from surgery, such as death, stroke or the need for re-hospitalization.
Patients involved in this study will be monitored for 10 years as part of the research study protocol. For more information about TAVR and the findings of this study, please visit “Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients” in The New England Journal of Medicine.