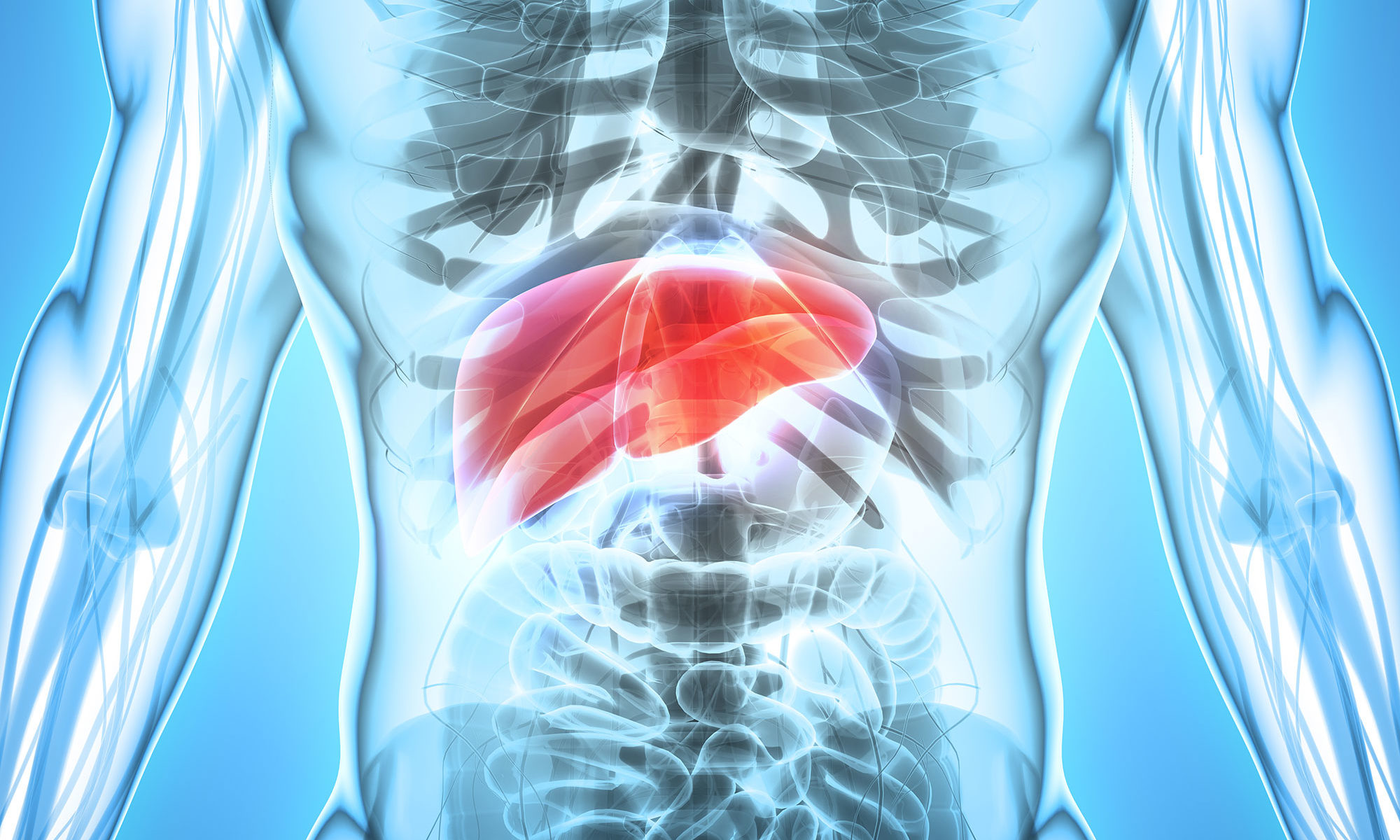
The opioid epidemic has resulted in thousands of deaths in people infected with acute hepatitis C (HCV) due to IV drug use. Because many of these people are undiagnosed, especially in high-risk populations, many organs from HCV-positive donors are still being procured. The development of highly effective direct-acting antiviral (DAA) therapy has made it possible to successfully transplant HCV-positive organs into HCV-negative recipients and treat the hepatitis C post transplant.
A “Prospective Multicenter Study of Early Antiviral Therapy in Liver and Kidney Transplant Recipients of HCV‐Viremic Donors,” confirms that this preemptive antiviral strategy is an effective way to expand the donor pool. Results of the study were published Sept. 14 in HEPATOLOGY, a journal of the American Association for the Study of Liver Disease.
“It’s the convergence of two phenomena: the opioid epidemic which has led to young people dying of drug overdoses after using IV drugs and becoming infected with HCV and the advent of highly effective antiviral medications,” says James Trotter, MD, medical director, transplant hepatology, for Baylor Scott & White Health, chair of the UNOS Liver and Intestine Transplantation Committee and co-author of the study. “This study shows that this is a good practice and validates what is becoming commonplace across the country.”
Six U.S. transplant programs prospectively treated 24 donor HCV positive/recipient HCV negative liver transplant (13) and kidney transplant (11) recipients with sofosbuvir‐velpastasvir for 12 weeks. Treatment was begun once viremia was confirmed post‐transplant, and the patients were judged to be clinically stable. Primary endpoints were sustained virologic response at 12 weeks post‐transplant (SVR12) and safety as assessed by proportion of treatment‐related adverse and serious adverse events. At the end of treatment, all liver transplant recipients were HCV RNA undetectable, while three of the kidney recipients still had detectable, but not quantifiable, viremia. All patients ultimately achieved SVR12.
“With this approach, patients now have access to a wider donor pool and can potentially receive a transplant much earlier than in the past,” says Johanna Bayer, MD, surgical director of the Baylor Scott & White All Saints Medical Center Transplant Program and co-author of the study. “Kidney transplant recipients may wait five or six years before being called in. The use of HCV positive organs is becoming a commonly used approach to getting patients transplanted earlier before they become too sick. As this study shows, we can safely use these organs and eradicate the virus after transplant.”